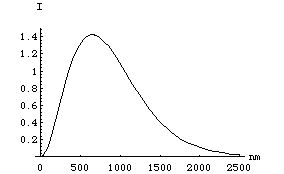
We call the 400 to 700 nm range of wavelengths "visible" because those are the wavelengths to which our eyes are sensitive. That range reflects the wavelengths of sunlight which reach the Earth's surface with sufficient intensity to excite the cells on our retinae. Minus absorption by O 2, H 2 O, etc., the "spectral distribution" (relative intensity as a function of wavelength) is a smooth curve with a peak at about 600 nm:

which is of course in the middle of the visible region. This spectrum is called a "black body" spectrum, and represents the spectrum of radiation of a "perfect" radiator (ie., the sun).
The physical process of seeing begins with the absorption of a photon by a pigment called "retinal". We can define a pigment as a substance which is "photoreactive", that is, which absorbs (and possibly re-emits) wavelengths in the visible range. The absorption causes a change in the geometry of the retinal (in the terms of organic chemistry, it changes from a "cis" to an "all-trans" configuration):
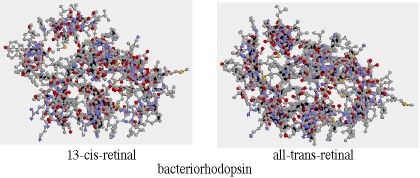
This change in geometry takes place in about 6 ps. It has the effect of activating an enzyme (phosphodiesterase) which in turn hydrolyzes hundreds of "cylic-GMP" molecules (guanosine 3' - 5' phosphate) which in turn effects the sodium channels in optic nerves, initiating a nerve impulse. (Note that at the level of proteins, geometry defines function; this business of shape-changing, as well as "key and lock" shape fitting, is a ubiquitous signaling mechanism in the world of molecular biology.) The specific protein to which the retinal is linked determines the range of wavelengths to which it is sensitive. Retinal linked to rhodopsin has a peak sensitivity of about 496 nm. Rhodopsin is the protein in human rod cells as well as in many photoactive organisms.
The cone cells of the human eye are sensitive to 3 wavelength ranges which the eye interprets as blue (narrow, with a peak near 419 nm), green (broader, with a peak near 531 nm) and red (also broad, with a peak near 558 nm, which is actually more like yellow!):
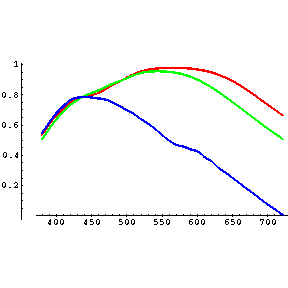
(from Vos, J. J. & Walraven, P. L.). All of the colors which your mind perceives are constructed from combinations of relative intensities of these three "wavelengths": red, green and blue are the only "signals" your brain receives from your eyes. Recall that the "intensity" of a sensation is proportional to the frequency of nerve impulses. If 650 nm photons hit your retinae, your brain will receive a mixture of green and red signals, with more red than green but not too many of either. This will be interpreted as red. Similarly, 475 nm photons will cause about equal numbers of blue and green signals, with only a few red; this will be interpreted as a sort of bluish-green. The number of signals for any one of these ranges depends on both the intensity of the light and the sensitivity at that wavelength. This leads to a vaguely disturbing contrast between sensation and perception: your eye sends only three kinds of signals to your brain, yet your brain "constructs" the full color spectrum of "reality" from them. Now you can understand why people can have violent disagreements over what color something is: no two people see exactly the same thing, yet we assume that color is something objective. Since most of us have similar sensitivities we can agree on primary colors, so the garment industry has nothing to fear.
"Scotopic" (dark-adapted) and "photopic" (light-adapted) sensitivities differ; scotopic peak sensitivity (of the rod cells) is at about 500 nm, while photopic sensitivity (of the cone cells) peaks at around 550 nm. Wavelengths shorter than 315 nm are absorbed by the cornea (causing injury) and do not reach the retina. Retinal sensitivity sometimes extends (with very low sensitivity) to 1000 to 1050 nm. Note that if your eyes were sensitive to much longer wavelengths you would look through a sort of "infrared fog", since you would see heat energy everywhere.
The next section is about infrared spectroscopy and degrees of freedom.
You can learn more about vision at the UCSD Color and Vision Database.
If you have stumbled on this page, and the equations look funny (or you just want to know where you are!), see the College Physics for Students of Biology and Chemistry home page.
©1996, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.