One of the key points in the last section is that photons can excite changes at the molecular level. That is, molecular bonds can absorb or emit photons while changing energy, just as atomic electrons do. This principle can be extended to wavelengths both above and below the visible spectrum:
| l (nm) | type of radiation | source |
|---|---|---|
| below 10 | x-rays, g rays | nucleii, electrons of large atoms |
| 10-400 | ultraviolet rays | atomic electrons |
| 400-700 | visible light | atomic electrons |
| 700-10K | infrared | molecular bond motions |
| 10K-1M | microwaves | rigid molecular motion |
| above 1M | radio waves | conduction electrons |
We will investigate g rays in the next chapter, and infrared and microwaves below. The important idea in this table is that the wavelength of radiation scales as the size of the source: smaller sources emit (and absorb) higher energy photons, while larger sources emit (absorb) lower energy photons. (Even though conduction electrons are small, they move over large conductors when generating radio waves; the waves therefore have long wavelengths and low energy.) We use this principle to deduce the effects of various radiations. For instance, we do not expect low frequency radio waves to be a significant cause of cancer, since they do not have sufficient energy to affect our body chemistry (which is determined by atomic electrons).
Any given molecule can change its energy in any of its "degrees of freedom". There is one degree of freedom per independent mode of motion, ie., translational (rigid "whole body" movement in the x, y or z directions), rotational (whole body rotation around one or two independent axes) or vibrational (oscillation of bond length or angle). These degrees of freedom are independent of coordinate system, so that rotations seen from two different points of view do not count separately. Also, we idealize the atom as a pointlike, structureless object for these purposes. For instance:
| molecule | translational | rotational | bond length | bond angle |
|---|---|---|---|---|
| C | 3 | 0 | 0 | 0 |
| O 2 | 3 | 1 | 1 | 0 |
| H 2 O | 3 | 2 | 2 | 1 |
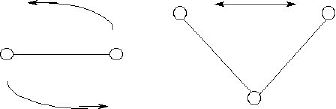
An atom, as a point object, cannot have rotational degrees of freedom: a point object has no dimension and hence no moment of inertia (nothing to rotate about an axis). Besides its three translational and one vibrational degrees of freedom, a diatomic molecule can only rotate rigidly about its center of mass, since it has no cross sectional dimension (a bond is idealized as having no thickness). For larger molecules, such as water, there can be an oscillation of the bond angle:

in addition to its two independent vibrational modes and its five rigid degrees of freedom (three translational and rotation around either bond). In general, any molecule (with more than two atoms) has 5 degrees of rigid motion freedom, one degree of bond length vibrational freedom for every bond, and one degree of bond angle vibrational freedom for every atom connecting two other atoms. You can see that the number of degrees of freedom for large molecules can be very great indeed!
Excitation of the vibrational modes of molecular bonds occur when the molecular orbitals absorb photons with infrared wavelengths:
| Group | Mode | Peak l (nm) |
|---|---|---|
| O - H | stretch | 2800 |
| N - H | stretch | 3000 |
| C - H | stretch | 3400 |
| C = O | stretch | 5800 |
| C = C | stretch | 6100 |
| N - H | rotation | 6500 |
| C - H | rotation | 7300 |
| H - C - H | scissors | 6800 |
Here the wavelength is given as the "peak" wavelength, which corresponds to the bond's "natural" mode of motion (see Chapter 9). The bonds can be excited at other wavelengths, but less easily. Note that more stable bonds have lower energy (higher wavelength) natural stretching modes, and visa versa. This is indicative of the strength of the bond, and its relative distaste for changing its length.
Microwave wavelengths correspond to whole molecule rotational modes. The microwave oven operates on the principle that there are a few rotational modes which are relatively easy to excite in water and fat molecules. Since these are the principle constituents of the foods we eat, the energy they absorb dissipates as heat (translational motion), and our food becomes hot. This is why water boils but the cup remains relatively cool, and why the cheese bubbles on the pizza before the crust is hot. We will meet these topics (heat and degrees of freedom) again in Chapter 8.
The next chapter is about nuclear physics.
If you have stumbled on this page, and the equations look funny (or you just want to know where you are!), see the College Physics for Students of Biology and Chemistry home page.
©1996, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.