Simplify the circuits pictured below as much as possible by identifying like components in series or parallel.
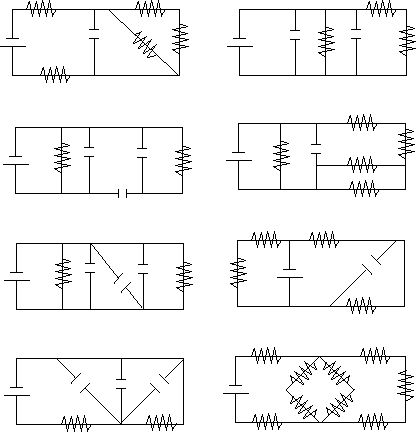
We will see in the next section that such components can be eliminated in favor of an equivalent component; ie., two resistors in parallel can be replaced by a resistor of a different value, etc. Note that not all pairs of like components can be combined and that at least one circuit cannot be simplified at all.
4-20 through 4-27:
Refer to the diagrams above. Ignore all of the capacitors. Assume that the batteries are all 12 V, and all of the resistors are 5 W. What is the voltage drop and current across each of the resistors?
4-28. Normal household current (120 V, 60 Hz alternating current) produces the following reactions for 1 second of skin contact:
What is the effect of a 1 second shock when
4-29. It is very important NOT to ground a patient (which would allow current to flow from a medical instrument through their body to the ground), since even very small currents (20 mA) can cause ventricular fibrillation when applied directly to the heart (ie., through a catheter). Consider a patient who is inadvertently grounded and whose resistance to ground is 1 KW due to the insertion of a catheter. Assume that an EKG monitor wire is leaking 30 mV through the patient's skin. Is the patient in danger?
4-30. The space shuttle often flies in an approximately circular orbit 300 km above the surface of the Earth, orbitting once every hour and a half. Compute the acceleration due to gravity in the shuttle. Why do the astronauts appear to be weightless?
4-31. Ben Franklin is credited with determining that electric charge comes in two varieties, which he astutely called "positive" and "negative" because they acted as opposites; the addition of one to the other results in zero charge. How could he be sure that there were only two? (Hint: consider the results of applying first one charge then another; does it depend on the order of application?) If there were actually three types of charge, which were not opposites but instead acted as primary colors (such that only the sum of all three is colorless, or neutral), how would he have discovered them?
If you have stumbled on this page, and the equations look funny (or you just want to know where you are!), see the College Physics for Students of Biology and Chemistry home page.
©1996, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.
Additional Problems
I (mA) Effect 1 awareness threshold 5 maximum harmless current 10-20 sustained muscular contraction 50 pain, possibly fainting and exhaustion 100-300 ventricular fibrillation 6000 temporary respiratory paralysis, possibly burns
Want some more practice with charges in a potential field?
Need more problems about chemical bonds?
How about problems about resistors and circuits?