Now that you think you understand something about physics, it's time for a curve ball.
If we perform the double slit diffraction experiment using a beam of electrons instead of light, we still get a diffraction pattern. The interpretation of this is that matter travels as a wave. But there's more. Recall that in Chapters 5 and 6 we discussed "particles" of electromagnetic radiation called photons. We can arrange for light to enter the slits one photon at a time, and we can do the same thing for electrons. In both cases, we still (over time) build up a diffraction pattern. One interpretation of this result is that a single photon or electron goes through both slits and interferes with itself. Thus the common statement that "matter acts as both a particle and as a wave."
If we can sometimes consider an electron to be a wave, what is its wavelength? Using Planck's constant and some dimensional reasoning, we can see that the "Compton" wavelength of a particle depends on its momentum:
So an electron travelling at 0.01 times the speed of light has a Compton wavelength of 2.4 Angstroms. This gives a theoretical limit to what can be seen using an electron microscope (the actual limit is much larger due to other considerations). Note that the wavelength is on the order of the diameter of an atom. Of course, we can obtain much larger wavelengths by using slower electrons, but in any case the wavelength will be very small. Using the results of the last section, we can see that if the slits are separated far enough to determine "which slit the electron went through", the diffraction pattern washes out and the electron seems to be a particle again: it goes through one slit or the other. So it acts like a wave only under those circumstances for which it is impossible to see it as a particle! And it acts like a particle only under those conditions for which it is impossible to see it act like a wave!
These experiments have resulted in a vast amount of cogitation and speculation ever since they were first performed. At this juncture, we still have no idea "what an electron really is" (or what anything else is, for that matter!). The common wisdom is that it "has both particle and wave characteristics". What this really means is that both the wave and particle models are flawed and/or incomplete models for subatomic particles. Neither is sufficient to capture the essence of the beast, and no single model has successfully done so to date.
While this is all very puzzling from a qualitative, conceptual viewpoint, there is a vast amount of experimental data which verifies the wave aspects of matter at the subatomic level. Orbital electrons are essentially three dimensional standing waves, and covalent bonds in solids are modelled as standing waves between the points of a lattice. Particles are often modelled successfully as wave "packets" (sums of waves of different frequencies), and the velocity of the particle is then equal to the group velocity of the packet envelope. Diffraction effects imply that matter waves are superposable (at least in the linear wave model, which is all anyone knows how to deal with). This implies that the Pauli Principle is the only thing that keeps us from passing through each other and the Earth! Perhaps one of the most mysterious phenomena is "tunneling", which is a natural result of the wave model of matter.
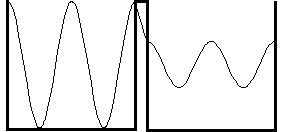
Classically, a dish at rest cannot spontaneously jump from one side of the sink to the other. If it doesn't have enough energy to get over the gravitational potential energy "barrier" that the middle wall of the sink represents, it will stay where it is. For an electron, this is another matter (pun intended). Quantization of wave number in the sink implies that it has a minimum nonzero energy; this is called its "zero-point" energy. Hence a quantum particle is never at rest (notice how we mix models that are essentially inconsistent!). There is a solution to the wave equation which is a standing wave on both sides of the sink, and an exponentially decreasing function through the wall between them:

So it is possible for an electron in one side of the sink to "spontaneously" (no one knows what happens during the transition) jump to the other side, even when it does not have enough energy to get across! If this tunneling seems impossible to you, consider the plug on the electric light you are using right now. It has a coating of copper oxide which provides an effective energy barrier to the conduction of electrons. Yet the light is on: the electrons are regularly tunnelling from the wall outlet into the electrical cord attached to the light!
We leave you with this further mystery in the world of physics (which is after all, the real world!) While physics, in company with mathematics, can make incredibly accurate predictions of a numerical nature about phenomena ranging from electrons to clusters of galaxies, we still do not understand qualitatively what matter or energy is, and why they must act the way they do.
This is the end of the hyperbook. Hope you have enjoyed it, and learned something in the bargain!
If you have stumbled on this page, and the equations look funny (or you just want to know where you are!), see the College Physics for Students of Biology and Chemistry home page.
©1996, Kenneth R. Koehler. All Rights Reserved. This document may be freely reproduced provided that this copyright notice is included.
Please send comments or suggestions to the author.